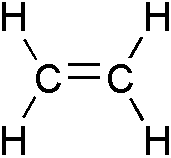
to start off, let's look at a visual!
( here's chemical structure of an ethylene)
A.K.A. ( also know as)
moving on..
- hydrocarbons with double bonds make up this family ( alkene )
- it's unsaturated
- they are also insoluble and flammable
- and the ending changes from ane to ene
Step 1: fine the longest chain
Step 2: find the # of carbon to get the lowest # for the start of the double bond; then place that number before the name
Step 3: name and number all the side groups and arrange them alphabethically.
FOR EXAMPLE:
CH3CH=CHCH3
- so, the longest chain is.. four. so that's butane
- now remember to change the ending into ene
- now the bond is located at the second place- so you call this.. 2-butane. (pretty easy ryt?) how about this one?give it a try. CH3 CH3
| |
CH3CH2CHCH2CH=CCH3since the double bond is at the end. you count starting from RIGHT to LEFT.
answer:
2,5-dimethyl-2-heptene Alkanes with two double bonds are called dienes, those with three are called trienes, and so on...
Now, when it comes to alkenes, some molecules will have the same structure but they have different geometry.
"Cis" and "Trans"
geometric isomerism among alkenes.
- this is used so we can differentiate 2 chemical formulas that are the same but are arranged differently.
- "cis" and "trans" are the two geometric isomers
- "cis" means on the same side
- "trans" means on the opposite ends
FOR EXAMPLE:
cis-2-butene
CH3 CH3 ( see how CH3 are on the same sides ?) \ / C = C / \ H H
trans-2-buteneCH3 H ( see how CH3 are on the opposite sides?) \ / C = C / \ H CH3
here;s a better visual picture (:
LEFT PICTURE: cis-2-butene RIGHT PICTURE: trans-2-butene **Note: that if the top and bottom are the same; there's no need to use cis or trans.** Alkynes now alkynes have the same rules as alkenes except they ends with ynecompounds with low polarityhave one or triple bondformula is CnH2n-2for example:
Here's a diagram for more examples of different types of alkyne and alkene's molecular formulas.still dont get it?here's some videos you can watch.
sry fellow bloggers but i have no joke for today. i dont feel like being funny today.so good day to u all!or should i say goodnyt!ta-taO.O




No comments:
Post a Comment