A group of organic molecules were having a party, when a group of robbers broke into the room and stole all of the guest's joules. A tall, strong man, armed with a machine gun came into the room and killed the robbers one by one. The guests were very grateful to this man, and they wanted to know who he was. He replied: My name is BOND, Covalent Bond.
Wednesday, December 15, 2010
Sunday, December 12, 2010
Lab 4C
We did a lab on Thursday and it was about the composition of the hydrate.
The purpose of the lab was to determine the percentage of water in an unknown hydrate, to determine the moles of water present in each mole of this unknown hydrate, when given the molar mass of the anhydrous salt and to find out the empirical formula of the hydrate.
So the procedure of this lab was:
- put on safety equipment (always the most important)
- set up the equipment (ring stand, ring, bunsen burner, pipestem triangle, crucible, and crucible tongs)
- turn on the bunsen burner to dry the crucible
- remove the burner and allow the crucible to cool for about 3 minutes
- determine the mass of the empty crucible
- place 1/3 full of hudrate and determine the mass (record it down)
- place the crucible and contents on the pipestem triangle and heat until the crucible is a dull red (maintain the temperature for 5 minutes)
- turn off and allow the crucible to cool for 5 minutes
- record the mass
- reheat the crucible for another 5 minutes, cool as before, and determine the mass (the mass should be the same as the first one)
- add water to the content of the crucible and note any changes that occur
Analysis of Results
1) The percentage of water in the hydrate is calculated by :
mass of water divided by mass of hydrate x 100%
2) The number of moles the anhydrous salt left behind is calculated by :
Mass after heating – mass of crucible = mass of anhydrous salt
Moles of anhydrous salt = mass of anhydrous salt x 1 mole = moles of anhydrous salt 120.4g
3) The number of moles of water removed by the heat is calculated by :
Mass of hydrate = mass of crucible & hydrate – mass of crucible
1) The percentage of water in the hydrate is calculated by :
mass of water divided by mass of hydrate x 100%
2) The number of moles the anhydrous salt left behind is calculated by :
Mass after heating – mass of crucible = mass of anhydrous salt
Moles of anhydrous salt = mass of anhydrous salt x 1 mole = moles of anhydrous salt 120.4g
3) The number of moles of water removed by the heat is calculated by :
Mass of hydrate = mass of crucible & hydrate – mass of crucible
mass of water = mass of hydrate – mass of salt
Moles of water = mass of water x 1 mole = moles of water
18g
4) The moles of water per mole of anhydrous salt is calculated by :
Moles of water divided by moles of salt is the ratio of water to anhydrous salt
5) The empirical formula of the hydrate is MgSO4 · 7H2O Magnesium Sulphate Heptahydrate.
Follow-Up Questions
1) SOURCES OF ERROR
- NOT HEATING IT TO BLUE FLAME
- HEATING IT WAY TOO LONG
- WRONG MEASUREMENT
- USING THE WRONG EQUIPMENT
2) Solution :
Na => 16.1g x 1 mole = 0.7 moles of Na x3 2 23g
C => 0.35 moles of C x3 1
O => 1.05 moles of O x3 3
O => 1.05 moles of O x3 3
Water => 3.5 moles of water x3 10
The empirical formula is Na2Co3 · 10H2O
3) Solution:
Molar Mass of Na2CO3 x 5.82g = 106g/mol x 5.82 = 2.16g
Molar Mass of Na2CO3 · 10H2O 286g/mol
Molar Mass of Na2CO3 · 10H2O 286g/mol
4) Solution:
Moles of Na 18.53g x 1 mole = 0.806 moles of Na 1 x 2 2 23g
S = 0.806 moles of S 1 x 2 2
O = 1.21 moles of O 1.5 x 2 3
O = 1.21 moles of O 1.5 x 2 3
H2O = 2 2.5 x 2 5
The empirical formula is Na2S2O3 · 5H2O.
VIDEOS!
Empirical Formula : Hydrate
Wednesday, December 8, 2010
The Empirical Formula of Organic Compounds
Finding the empirical formula of an organic compound (any substance with carbon in it) is pretty similar to calculating the empirical formula to other substance. Except it has one additional step at the end.
It can be found by:
- Burning the compound (reacting it with O2)
- Collecting and weighing the products
- The mass of the products, the moles of each element in the original organic compound can be calculated
There are 4 steps you need to follow if you want to find the empirical formula correctly.
Here is an example question and the steps that follow.
What is the empirical formula of a compound that when a 5g sample is burned produces 15g of CO2 and 8.18g of H2O?
Step 1
Calculate the moles of CO2 + H2O produced.
mol CO2 = 15g CO2 x (1 mol CO2/44.0g CO2) = 0.341 mol
mol H2O = 8.18g H2O x (1 mol H2O/18.0g H2O) = 0.454 mol
Step 2
Find the moles of C + H in CO2 and in H2O.
mol C = 0.341 mol CO2 x (1 mol C/1 mol CO2) = 0.341 mol C
mol H = 0.454 mol H2O x (2 mol H/ 1 mol H2O) = 0.908 mol H
Step 3
The ratio of moles of C to moles of H in the organic compound is 0.341: 0.908.
Therefore the formula could be: C0.4341H0.908 BUT the ratio must be whole numbers.
Step 4
Change the ratio to a whole number ration by multiplying in turn by 2/2, 3/3, 4/4 until you get a whole number ratio.
Ratio of moles = 0.908 mol H/ 0.341 mol C = (2.66/1) x 3/3 = 8/3
The empirical formula = C3H8
It can be found by:
- Burning the compound (reacting it with O2)
- Collecting and weighing the products
- The mass of the products, the moles of each element in the original organic compound can be calculated
There are 4 steps you need to follow if you want to find the empirical formula correctly.
Here is an example question and the steps that follow.
What is the empirical formula of a compound that when a 5g sample is burned produces 15g of CO2 and 8.18g of H2O?
Step 1
Calculate the moles of CO2 + H2O produced.
mol CO2 = 15g CO2 x (1 mol CO2/44.0g CO2) = 0.341 mol
mol H2O = 8.18g H2O x (1 mol H2O/18.0g H2O) = 0.454 mol
Step 2
Find the moles of C + H in CO2 and in H2O.
mol C = 0.341 mol CO2 x (1 mol C/1 mol CO2) = 0.341 mol C
mol H = 0.454 mol H2O x (2 mol H/ 1 mol H2O) = 0.908 mol H
Step 3
The ratio of moles of C to moles of H in the organic compound is 0.341: 0.908.
Therefore the formula could be: C0.4341H0.908 BUT the ratio must be whole numbers.
Step 4
Change the ratio to a whole number ration by multiplying in turn by 2/2, 3/3, 4/4 until you get a whole number ratio.
Ratio of moles = 0.908 mol H/ 0.341 mol C = (2.66/1) x 3/3 = 8/3
The empirical formula = C3H8
Sunday, December 5, 2010
JOKE OF THE DAY
> >A psychotic chemist came home from work and had a big
> >fight with his wife. In the heat of the moment, he
> >grabbed a bottle of some lethal chemical substance and
> >forced her to drink it while he screamed: " Die Ethyl,
> >die". The wife dropped dead on the floor and the
> >neighbors who were watching the scene, decided to call
> >the police. The policemen arrived and arrested the
> >chemist. One of them asked: Was there any reason for
> >you to kill your wife? The chemist replied: " There
> >was no chemistry between us. We never bonded well
> >although we tried.In the compound where we lived, our
> >temperaments collided. She always responded negatively
> >to my comments. Our relationship was unstable. There
> >was no possible solution. She had an attitude and I
> >was explosive. Finally, I overreacted. But now I'm
> >glad it's over. I'm in equilibrium again.I will feel
> >free even behind the irons."
> >fight with his wife. In the heat of the moment, he
> >grabbed a bottle of some lethal chemical substance and
> >forced her to drink it while he screamed: " Die Ethyl,
> >die". The wife dropped dead on the floor and the
> >neighbors who were watching the scene, decided to call
> >the police. The policemen arrived and arrested the
> >chemist. One of them asked: Was there any reason for
> >you to kill your wife? The chemist replied: " There
> >was no chemistry between us. We never bonded well
> >although we tried.In the compound where we lived, our
> >temperaments collided. She always responded negatively
> >to my comments. Our relationship was unstable. There
> >was no possible solution. She had an attitude and I
> >was explosive. Finally, I overreacted. But now I'm
> >glad it's over. I'm in equilibrium again.I will feel
> >free even behind the irons."
Empirical and Molecular Formula
Empirical Formula gives the lowest term ratio of atom in the formula
Finding the empirical formula is somewhat the reverse of finding percentage composition. ( see below)
First you will be given the percentages of each element in a particular compound.
Assuming that you will always be given a 100 gram sample we will convert these percentages to grams. The next step is to find the ratio of moles of each element and then calculate the simplest ratio of subscripts that maintain that ratio.
It is much easier done than said!
Lets start with this data: 36.5% Na, 25.4% S, and 38.1% O
First, convert each percentage to grams : 36.5 g Na, 25.4 g S, 38.1 g O.
Next, divide each by the grams/mole of that element:
Na: 36.5 g / 23.0 g/mol = 1.58 mol Na
S: 25.4 g / 32.1 g/mol = 0.791 mol S
O: 38.1 g / 16.0 g/mol = 2.38 mol O
Now we can set up the ration of moles of each element:
Na S O
1.58 0.791 2.38
To convert these decimal numbers into whole numbers and maintain the same ration between them, just divide each by the smallest of the subscripts.
1.58/0.791
0.791/0.791
2.38/0.791
Our final formula would look like: Na2SO3 ( sodium sulfite)
MORE EXERCISES!!! :)
Molecular Formula is a multiple of the empirical formula
Molecular Formula = Molar Mass / Molar Mass of empirical formula
To find the molecular formula, first find the empirical formula for the data you have been given. Then compare the formula mass for your empirical formula with the formula mass for the molecular formula. You may have to multiply the subscripts in your empirical formula by some factor.
Sample Problem.
Given: 38.7% C, 9.70% H, 51,6% O and a molecular formula mass of 62.0 find the molecular formula:
First, find the empirical formula ===> CH3O
Next find the formula mass: C 1 x 12.0 = 12. 0
H 3 x 1.01 = 3.03
O 1 x 16.0 = 16.0
Formula mass = 31.0
Now divide the molecular mass by this formula mass : 62.0 / 31.0 = 2
Multiply each subscript in your formula by this factor ( 2 here)
Your final molecular formula: C2H6O2
To check your answer find the formula mass of your final formula. It should be the same as the molecular formula mass.
Practice:
Given the following data, find the correct molecular formula:
34.6% C , 9.00% H, 36.4% O and a molecular mass of 176
The correct answer would be: C8H16O4
The empirical formula would be: C2H4O with a formula mass of 44
The multiplication factor would be 4 ( 176/44.0)
ONE MORE TIME!
VIDEO!!!!!!!!!!!!!! (:
Thursday, December 2, 2010
Percentage Composition
The percent composition (percentage composition) of a compound is a relative measure of the mass of each different element present in the compound.
To calculate the percent composition (percentage composition) of a compound
Example:
NaCl
The answers above are probably correct if %Na + %Cl = 100, that is,
39.34 + 60.66 = 100.
28.19 + 8.11 + 20.77 + 42.93 = 100
How to calculate percent composition?
Hmm...
(:
To calculate the percent composition (percentage composition) of a compound
- Calculate the molecular mass MM, of the compound
- Calculate the total molar mass of each element present in the formula of the compound
- Calculate the percent composition (percentage composition)
Example:
NaCl
- Calculate the molecular mass (MM):
MM = 22.99(Na) + 35.45(Cl) = 58.44g/mol - Calculate the total molar mass of Na present:
1 Na is present in the formula, molar mass = 22.99g/mol - Calculate the percent by weight of Na in NaCl:
%Na = (molar mass Na ÷ MM) x 100 = (22.99 ÷ 58.44) x 100 = 39.34% - Calculate the total mass of Cl present:
1 Cl is present in the formula, molar mass = 35.45g/mol - Calculate the percent by weight of Cl in NaCl:
%Cl = (molar mass Cl ÷ MM) x 100 = (35.45 ÷ 58.44) x 100 = 60.66%
39.34 + 60.66 = 100.
Calculate the percent by weight of each element present in ammonium phosphate [(NH4)3PO4]
- Calculate the molecular mass (MM) of (NH4)3PO4:
MM = 3x[14.01 + (4 x 1.008)] + 30.97 + (4 x 16.00) = 3 x [14.01 + 4.032] + 30.97 + 64.00 = (3 x 18.042) + 30.97 + 64.00 = 54.126 + 30.97 + 64.00 = 149.096 - Calculate the total mass of N present:
3 N are present, mass = 3 x 14.01 = 42.03 - Calculate the percent by mass of N present in (NH4)3PO4:
%N = (mass N ÷ MM) x 100 = (42.03 ÷ 149.096) x 100 = 28.19% - Calculate the total mass of H present:
12 H are present in the formula, mass = 12 x 1.008 = 12.096 - Calculate the percent by mass of H present in (NH4)3PO4:
%H = (mass H ÷ MM) x 100 = (12.096 ÷ 149.096) x 100 = 8.11% - Calculate the total mass of P present:
1 P is present in the formula, mass = 30.97 - Calculate the percent by mass P in (NH4)3PO4:
%P = (mass P ÷ MM) x 100 = (30.97 ÷ 149.096) x 100 = 20.77% - Calculate the total mass of O present:
4 O are present in the formula, mass = 4 x 16.00 = 64.00 - Calculate the percent by mass of O in (NH4)3PO4:
%O = (mass O ÷ MM) x 100 = (64.00 ÷ 149.096) x 100 = 42.93%
28.19 + 8.11 + 20.77 + 42.93 = 100
How to calculate percent composition?
Hmm...
(:
Tuesday, November 23, 2010
Mole Conversion
Recall: 1 mol = 6.022 E 23 units of substance
Conversion between particles and mole
To do this type of questions, you have to know the molar mass of the substance. Using the periodic table we can find the molar mass, or the mass of a mole of a substance. The unit of the molar mass is grams/ mol
the following question is more difficult and a mole map can help you understand it.
Conversion between particles and mole
- Particles to mole
- multiplied particle/ formula units/ molecules by 1 mol/6.022 x 10^23
- Example: How many moles of barium nitrate (BaNO3) contain 6.80 x 10^24 formula units?
- Well, to do this, divide 6.80 x 10^24 by Avagadro's number, 6.022 x 10^23, to get 11.29 moles.
- multiplied mole by 6.022 x 10^23/ 1 mol
- Example: Determine the number of atoms that are in 0.58 mol of Se
- If you're thinking you multiply .58 moles by 6.022 x 10^23 atoms/mol, you're absolutely right. This yields the answer of 3.49 x 10^23 atoms of Se.
To do this type of questions, you have to know the molar mass of the substance. Using the periodic table we can find the molar mass, or the mass of a mole of a substance. The unit of the molar mass is grams/ mol
- Moles to grams
- multiply moles by _grams / 1mol
- Example: how many grams are there in 2.04 moles of Carbon?
- To do this question, first you have to find the molar mass of C and it is 12.0g/ mol. Then, you multiply 2.04 mole by 12.0g/ 1 mol and get the answer of 24.5g of C
- multiply grams by 1 mol/ _grams
- Example: how many moles are in 3.45 g of Carbon?
- Well, the molar mass of Carbon is 12.0 g/ mol so you multiply 3.45 g by 12.0g/ mole and get the answer of 0.288 mol.
the following question is more difficult and a mole map can help you understand it.
- Particles to mass
- To do this type of question:
- Find the moles of the item you wish to convert
- Determine the molar mass of the item you wish to convert. (set up the conversion factor - this time moles are on the bottom)
- Set up the equation and solve
- Example: what is the mass of 2.78 x 10^ 22 Fe atoms?
- To do this, we multiply 2.78 x 10^ 22 by 1 mol/6.022 x 10^23 and times the molar mass: 55.8 g/ 1 mole to the answer and get 2.58 g Fe
- The rules for this type of questions are
- Find the mass of the item you wish to convert
- Determine the molar mass of the item you wish to convert.
- Set up the equation and solve.
- Example: how manu atoms of Iron in 20.0 g of Iron?
- For this question, we time 20.0 g by the molar mass of the Iron : 1 mol/ 55.8 g, then we multiply the result 6.022 x 10^23/ 1 mol and get the result of the number of atoms, which is 2.16 x 10^ 23 atoms of Fe
Saturday, November 20, 2010
Atomic Mass, Formula Mass, and Molar Mass
Yay it was snowing last night :). Who doesnt love snow??? (besides people who drive.) Anyways, this blog is for science... So, the last 2 classes we learnt about different masses and how to find them. Here are the notes to remind you of what we know so far.
Avogadro's Hypothesis
- The volumes of different gases at the same temperature and pressure have the same amount of particles.
- If they have the same number of particles, mass ratio is caused by the mass of the particles
- This principle is used for the relative mass of all the atoms on the periodic table
Avogadro's Number (not phone number :p)
-The number of particles in 1 mole of any amount of substance is 6.022x10²³ particles/mole
- The mole allows chemists to count atoms and molecules a lot easier.
-Atoms can also be counted by weighing

Relative Mass
-It is the mass of the different elements that make up a particular formula.
Atomic Mass
- The mass of an atom of a chemical element expressed in atomic mass units. (Its found on the periodic table to make it easier.)

* amu stands for atomic mass units
Formula Mass
- All atoms of a formula of an ionic compound (in amu)
ex: Potassium has 39.1 atomic mass and Fluorine has 19.0. That would mean KF (potassium fluoride) would equal to 58.1 amu. Just need to add the atomic mass.
Molecular Mass
- All atoms of a formular in a covalent compound. (in amu)
ex: O2 would be 16.0u + 16.0u = 32.0u
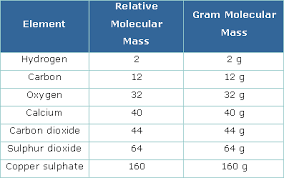
Molar Mass
- The mass of 1 mole (6.022x10²³ particles) in a substance and is the same numerical value of atomic mass, formula mass, or molecular mass but expressed in GRAMS PER MOLE.
ex: The molecular mass of oxygen is 16.0u and the molar mass of oxygen is 16.0g/mole.

That's all for today :)
HAVE FUN IN THE SNOW!!

Avogadro's Hypothesis
- The volumes of different gases at the same temperature and pressure have the same amount of particles.
- If they have the same number of particles, mass ratio is caused by the mass of the particles
- This principle is used for the relative mass of all the atoms on the periodic table
Avogadro's Number (not phone number :p)
-The number of particles in 1 mole of any amount of substance is 6.022x10²³ particles/mole
- The mole allows chemists to count atoms and molecules a lot easier.
-Atoms can also be counted by weighing

Relative Mass
-It is the mass of the different elements that make up a particular formula.
Atomic Mass
- The mass of an atom of a chemical element expressed in atomic mass units. (Its found on the periodic table to make it easier.)
* amu stands for atomic mass units
Formula Mass
- All atoms of a formula of an ionic compound (in amu)
ex: Potassium has 39.1 atomic mass and Fluorine has 19.0. That would mean KF (potassium fluoride) would equal to 58.1 amu. Just need to add the atomic mass.
Molecular Mass
- All atoms of a formular in a covalent compound. (in amu)
ex: O2 would be 16.0u + 16.0u = 32.0u
Molar Mass
- The mass of 1 mole (6.022x10²³ particles) in a substance and is the same numerical value of atomic mass, formula mass, or molecular mass but expressed in GRAMS PER MOLE.
ex: The molecular mass of oxygen is 16.0u and the molar mass of oxygen is 16.0g/mole.

That's all for today :)
HAVE FUN IN THE SNOW!!

Wednesday, November 10, 2010
Joke of the day!
A small piece of sodium that lived in a test tube fell in love with a Bunsen burner. "Oh Bunsen, my flame," the sodium pined. "I melt whenever I see you," The Bunsen burner replied, "It's just a phase you're going through."
GRAPHING!! (:
So.... for 2 classes we did graphs by using the microsoft excel.
Graphs that were taken includes: Density of Water, Density of Hot water, Temperature Vs volume of gas and lastly Mass Vs Volume.
How to graph?
As an example, here's how to graph the Density of Water.
STEPS:
- open excel
- plug in all the numbers for volume(mL) and mass (g) { make sure its separated into 2 columns}
- highlight both column then click "chart"
- choose "scatter"
- lable: title, subtitle, trend line type ( highlight + click "show formula" to get the formula) , x and y value
- click "add decimal place ( or something)" if the # keeps rounding itself to 1 ( this refer to the last 2 graph that we did)
- NOW, you get to design your graph. BE creative. Do ANYTHING you want. Lol
Question: Which one has a higher density? Cold water or Hot water?
Answer: Cold water, because as you heat water its molecules gain kinetic energy and the water becomes much less denseVIDEO!
.. on Water Density.Thursday, November 4, 2010
Lab 2E
Lab we did on Wednesday was about Density. The purpose of this lab was to calculate the thickness of a sheet of aluminum foil and express the answer in terms of proper scientific notation and significant figures
The procedure of this lab:
Experimental Error = |actual-experimental| /actual x 100%
the smaller experimental error percentage we get, the more accurate the result is
The procedure of this lab:
- Get 3 rectangular pieces of aluminum oil and label 1, 2, and 3
- Measure the length and width (using scientific notation)
- find the mass with centigram balance
- compare and discuss the result
- density = mass/ volume
- volume = width * length* height
Experimental Error = |actual-experimental| /actual x 100%
the smaller experimental error percentage we get, the more accurate the result is
Tuesday, November 2, 2010
Density
What is DENSITY ?
DENSITY is a physical property of matter, as each element and compound has a unique density associated with it. Density defined in a qualitative manner as the measure of the relative "heaviness" of objects with a constant volume.Density may also refer to how closely "packed" or "crowded" the material appears to be.
To calculate:
The units of density are typically g/cm3, but they can also have other designations that may be more convenient. The densities of various materials range from large values for heavy metals to very small values for gases.
DENSITY is a physical property of matter, as each element and compound has a unique density associated with it. Density defined in a qualitative manner as the measure of the relative "heaviness" of objects with a constant volume.Density may also refer to how closely "packed" or "crowded" the material appears to be.
To calculate:
| Density = | | g/mL |
| |
The units of density are typically g/cm3, but they can also have other designations that may be more convenient. The densities of various materials range from large values for heavy metals to very small values for gases.
In most cases, the density of an object can be used to predict whether it is a solid, liquid or a gas.
- Solids generally have a density greater than water (1 g/cm3). For example, aluminum has a density of 2.7 g/cm3. Yet, oak (0.75 g/cm3) is a solid and floats in water. Some exceptions are most varieties of wood, many plastics, and pumice.
- Liquids generally have a density near 1 g/cm3, with some being slightly above and some being slightly below. An exception is mercury (Hg), or quicksilver, which has a density of 13.6 g/cm3.
Matter with smaller density will float on top of the matter with bigger density.
The densities for some common substances are:
| Substance | Density (gm/cu.cm) |
| Air | 0.0013 |
| Wood (oak) | 0.85 |
| Water | 1.00 |
| Ice | 0.93 |
| Aluminum | 2.7 |
| Lead | 11.3 |
| Gold | 19.3 |
| Ethanol | 0.94 |
| Methanol | 0.79 |
Monday, November 1, 2010
Accuracy, Precision, and Uncertainty
It is true that we cannot measure things exactly (unless we're robots...) however that doesn't mean we can't give it our best. Here are a few notes we took last class, explaining the uncertainties of measurements.
- Precision: How reproducible a measurement is compared to other similar measurements.
- Accuracy: How close the measurement (or average measurement) comes to the accepted or real value.
Measurement and Uncertainty
- No measurement is exact. Only best estimates which has a some degree of uncertainty.
-Only when we count do we get an exact number (ex: There are 28 students in this class. There cannot be
28.5... that would be just weird.)
Absolute Uncertainty
-Uncertainty is expressed in the units of measurement not ratio
Method 1: Make 3 measurements, calculate the average (Make sure to take out the measurements that are "different" from the others. Ex: 2.3, 2.32, 2.46, 2.33). The absolute uncertainty = largest difference between the average and lowest/highest reasonable measurement.
Method 2: Determine the uncertainty of each instrument. Always measure to the best precision that you can. Therefore you should estimate to a fraction 0.1 of the smallest segment on the instrument scale. (On your ruler the smallest division = 1mm. Your best precision should be to break this into 10 equal pieces.)

Relative Uncertainty and Significant Figures
-Relative uncertainty = absolute uncertainty divided by estimated measurement
-Relative uncertainty can be expressed
- In percent %
- or using significant figures (sig. figs.)
- The number of sig. figs. = relative uncertainty: The last digit in a measurement is uncertain as it could be one digit higher or lower very easily.
Wednesday, October 27, 2010
Joke of the day :D
A neutron walks into a bar. He asks the bartender, "How much for a beer?" The bartender offers him a warm smile and says, "For you, no charge"
Tuesday, October 26, 2010
Significant Figures!
One of the great things about science is that nobody's perfect (for some of you, that may take a little getting used to!)No measuring device can measure any quantity exactly, so one must remember the uncertainty in every measurement when using them in calculations.
Digits in a measurement that are important for it are called significant figures (or significant digits). Using them keeps us honest, because we are prevented from seeming overly precise in our work.
In order to present results with the proper precision, we need to know how many significant figures are present in each number we use in a calculation. There are four basic rules:
(the answers are 1, 2, 2, 2, 3, 3, 5, 2, 3).
When adding or subtracting, the answer can only be as precise as the least precise number used. For example, a 250 pound person who has a hair pulled out (say, 0.001 pounds) still weighs 250 pounds. That's because the last significant digit is the 5 (in the tens place), and everything after that is not even estimated. So you have no idea how many ones of pounds the guy weighs, or how many tenths, or hundredths, or thousandths. Therefore, you have no idea what from to subtract 0.001 pounds. So he still weighs 250 pounds.
When multiplying or dividing, the answer has the same number of significant digits as the number used with the least. For instance, there is a quick estimation of pi (p) as 22¸7. On a calculator, that gives 3.1428571423, which is pretty close. But if you had measured a circle as 22 feet around and 7 feet across, the answer must be rounded to "3" to match the least number of significant digits.
*remember*
when multiplying/dividing, round it to the fewest number significant digits.
VIDEOS! :) .. on significant figures
Digits in a measurement that are important for it are called significant figures (or significant digits). Using them keeps us honest, because we are prevented from seeming overly precise in our work.
In order to present results with the proper precision, we need to know how many significant figures are present in each number we use in a calculation. There are four basic rules:
- Zeroes in the beginning of a number never count.
- Zeroes at the end of a number count only if there is a written decimal point.
- The digits 1 - 9 always count.
- Zeroes between the digits 1 - 9 always count.
(the answers are 1, 2, 2, 2, 3, 3, 5, 2, 3).
When adding or subtracting, the answer can only be as precise as the least precise number used. For example, a 250 pound person who has a hair pulled out (say, 0.001 pounds) still weighs 250 pounds. That's because the last significant digit is the 5 (in the tens place), and everything after that is not even estimated. So you have no idea how many ones of pounds the guy weighs, or how many tenths, or hundredths, or thousandths. Therefore, you have no idea what from to subtract 0.001 pounds. So he still weighs 250 pounds.
When multiplying or dividing, the answer has the same number of significant digits as the number used with the least. For instance, there is a quick estimation of pi (p) as 22¸7. On a calculator, that gives 3.1428571423, which is pretty close. But if you had measured a circle as 22 feet around and 7 feet across, the answer must be rounded to "3" to match the least number of significant digits.
*remember*
when multiplying/dividing, round it to the fewest number significant digits.
VIDEOS! :) .. on significant figures
Tuesday, October 19, 2010
Lab 3B Summary
Today, we did a lab, Separation of a Mixture by Paper Chromatography.
Paper chromatography is a modern method used separate mixtures. Paper chromatography uses paper as the stationary phase and a liquid solvent as the mobile phase.
The purposes of this lab were to separate mixture of food colorings into their components and to identify the components of mixtures by means of their Rf values.
Materials we need: 3 large testtubes, 3 Erlenmyer flasks, 3 strips of 22 chromatography papers, food colourings, a pencil, a ruler and a calculator
The procedures of this lab would be:
Part I
So, Here's a great video about color chromatography. Watch it and you would know more about this lab!!

Paper chromatography is a modern method used separate mixtures. Paper chromatography uses paper as the stationary phase and a liquid solvent as the mobile phase.
The purposes of this lab were to separate mixture of food colorings into their components and to identify the components of mixtures by means of their Rf values.
Materials we need: 3 large testtubes, 3 Erlenmyer flasks, 3 strips of 22 chromatography papers, food colourings, a pencil, a ruler and a calculator
The procedures of this lab would be:
Part I
- draw a line across each strips of chromatography paper 4.0 cm from one end, then trim the end of the strip
- place 2.0 cm deep water in each testtube
- spot one strip with the color, label, and insert the strip in test tube A
- observe what happens
- identify two front on the paper after the next 10 min
- start part II as the movement slow down
- after 20 min, remove the strip and immediately draw a pencil line across the top edge
- measure d2 and d1 and record in Table 1 and 2
- clean up
- spot the second strip of CP with a sample of green food coloring, and spot the third CP with an unknown mixture
- insert the strips and follow the same procedures in part 1
- record data
So, Here's a great video about color chromatography. Watch it and you would know more about this lab!!
 | |

Sunday, October 17, 2010
Separation Techniques
So Last class we did a notes from Miss Chen Notes. Here's all the info .
*Basis for separation : Different components , Different properties.
*Strategy: Devise a process that discriminates between components with different properties..
*Basis for separation : Different components , Different properties.
*Strategy: Devise a process that discriminates between components with different properties..
High Density/Low Density
Volatile/nonvolatile
Soluble/Insoluble
...
Separation
*Components in a mixture retain their identities: mixtures components have different porperties
Some Basic Technic:
-Filtration: Select component by particle size.
-Floatation: Select component by density
-Crystallization & Extraction: Select component by Solubility
-Distillation: Select component by boiling point.
Hand Separation & Evaporation
-Hand separation (solids and solids)
*mechanical mix or heterogeneous mix can be separate by using magnet or sleeve
-Evaporation (solid dissolved in liquid solution)
-Boil away the liquid and the solid remains.
Filtration: (solids(not dissolved)and liquids)
-Pass a mix that contains solid particles through a porous filter.
-If pores are smaller than particles,solid particles stay on filter and liquid/gaseous components pass through often used after separate by precipitation
-Use filter paper- residue left in
Crystallization (solid in liquid)
-Precipitation: Converse solute to solid form by chemical or physical change.
-Solids are separated by filtration or floatation.
-Evaporate or cool - solid comes out as pure crystal.
Gravity Separation: (solids based on gravity)
A centrifuge whirls the test tube around at high speeds forcing the denser materials to the bottom. Workds best for small volumes.
Solvent Extraction
-Component moves in a solvent shaken with mixture.
-Mechanical Mixture (solid and Solid) use liquid to dissolve one solid and the other one dissolved.
-Solution: Solvent is insoluble with solvent already present. Solvent dissolves one or more substances and leaves unwanted substances behind.
Distillation (Liquid in Liquid Solution)
-heating a mixture can cause low-boiling components to volatilize (vaporize)
-Distillation is collecting and condensing volatilized component.
-Liquid lowest boiling temperature , always boils first.
Chromatography
-Flow the mixtures over a materials that retains some components more than others
-Mobile phase sweeps the sample over a stationary phase (as the wind sweeps the swarm over the flower bed)
-Separate very complex mixtures
Sheet Chromatography
-Paper chromatography (PC)
Liquid soaked into a sheet in stationary phase ,some take longer than others
-Thin layer chromatography (TLC)
Stationary phase is a thin layer of absorbent (Al2O3 or SiO2) coating a sheet of plastic bond the absorbant strongly and other weakly.
Solvent Extraction
-Component moves in a solvent shaken with mixture.
-Mechanical Mixture (solid and Solid) use liquid to dissolve one solid and the other one dissolved.
-Solution: Solvent is insoluble with solvent already present. Solvent dissolves one or more substances and leaves unwanted substances behind.
Distillation (Liquid in Liquid Solution)
-heating a mixture can cause low-boiling components to volatilize (vaporize)
-Distillation is collecting and condensing volatilized component.
-Liquid lowest boiling temperature , always boils first.
Chromatography
-Flow the mixtures over a materials that retains some components more than others
-Mobile phase sweeps the sample over a stationary phase (as the wind sweeps the swarm over the flower bed)
-Separate very complex mixtures
Sheet Chromatography
-Paper chromatography (PC)
Liquid soaked into a sheet in stationary phase ,some take longer than others
-Thin layer chromatography (TLC)
Stationary phase is a thin layer of absorbent (Al2O3 or SiO2) coating a sheet of plastic bond the absorbant strongly and other weakly.
Thursday, October 14, 2010
Naming Acids
Last class we learnt about Acids and their formulas. You may not know how to determine an acid if you just saw their formula, but that's what these notes are for. Once you're done reading these notes, (hopefully) you'll be an expert at naming acids and writing their formulas. If not, just ask Ms. Chen for help :p

First of all, we need to know how an acid is formed.
Acids
An ACID is formed when a compound composed of HYDROGEN ions and a negatively charged ion are dissolved in water. (Aqueous -aq-)
- Ions separate when dissolved in water
- H ion joins with H2O (water) to form H3O (Hydronium Ion)
Ex. H + Cl --> HCl(g)
HCl(g) + H2O(l) --> H3O(aq) + Cl(aq)
Naming Acids Guidelines (Simple Acids)
1. Use "hydro" as the beginning
2. Last syllable of the non metal is dropped and replaced with "-ic."
3. Add "acid" at the end.
* ____ide -> hydro____ic acid
Example. Name the SimpleAcids:
1. HF(aq) - Hydrofluoric acid
2. HCl(aq) - Hydrochloric acid
3. Hbr(aq) - Hydrobromic acid
4. HI(aq) - Hydroiodic acid
Naming Complex Acids
1. -ate replace with -ic
-ite replace with -ous
2. "Acid"at the end of the name.
Example. Name the Complex acids
1. HCH3COO(aq) - Acetic Acid
2. HClO3(aq) - Chloric Acid
3. HNO2(aq) - Nitrous Acid
4. HClO4(aq) - Perchloric Acid

Here's a website for you to practice what you've learned.
Have fun :)
Thursday, October 7, 2010
Writing and Naming Ionic and Covalent Compounds
Okay, so today we did a review on Ionic and Covalent compounds which we all learned from last year. If you dont recall any of the terms. Well then here it is.
Ionic Compounds
- contain electrically charged particles called ions
- ions can be positive or negative
- they attract each other strongly because of their opposite charges
- the number of positive charges must equal the number of negative charges in a compound
For example:
iron (III) sulfide
Example:
Covalent Compounds
- share electrons
- non-metal with non-metal
- all covalent compounds have two word names
- the first word correspond to the first element in the formula and the second corresponds to the second element in the formula except it ends with "ide"
For example:
 | |
| Name: Carbon Dioxide |
 |
| Name: Hydrogen Fluoride |
*NEED TO KNOW*
Diatomic molecules [highlighted in pink]
VIDEOS!
Covalent Bonding ... Or not!
( Examples of ionic and covalent bonding)
Tuesday, October 5, 2010
Lab 2B
Today, we had a lab. It's on heating and cooling processing. The purpose of this lab is to investigate the heating and cooling process, and to determine and compare two of them. We did the cooling process first and here is the procedure:
- put on safety equipment
- decide the roll (observer or recorder), prepare table 1
- obtain a test tube consisting of a themometer, remove the cotton plug and save it
- put 300mL 30-35'C water in a 400 mL beaker
- set up a ring stand, a buret clamp to hold the test tube
- put the testube assembly into cold water
- record the temperature every 30s
- record when the solidification begins and ends and other observations
- stop when the temperature reach 25'C
- change the roll
- turn the plate on and raise the temperature to 55'C and 60'C
- turn the hot plate to low heat when it reaches 55'C
- record the temperature in table 1 and every 30s after that
- readings should continue until it reaches 50'C
- record the times when melting beings and ends.
- reseal the test tube assembly with the cotton plug and return all the equipment
- qash your hands with soap and water before leaving
Monday, October 4, 2010
Joke of the day
Two atoms are walking down the street.
Says one atom to the other, "Hey! I think I lost an electron!"
The other says, "Are you sure??"
"Yes, I'm positive!"
hahaa .. (ya i know. its not that funny)
Sunday, October 3, 2010
Summary of 2.3, 2.4, and 2.7
Finally! It's the weekend now we can relax and do our science review!! Oh ya..science review. Anyways, here's a short summary of the pages assigned by Ms.Chen to read and take notes on. Enjoy.
2.3 Characteristics of Pure Substances p.28
- Pure substances have a constant boiling point
- Mixtures ordinarily don't have a constant boiling point (however, a few do)
- Freezing Point: The temperature a liquid changes to solid
- Melting Point: Temperature a solid turns into a liquid
2.4 Chemical and Physical Changes p.30
- Density: Property of matter that describes its mass per unit volume
- Sugar and Baking powder do not have a melting/freezing point because a new substance is formed when
heated or cooled.
- Chemical Change: Changes that produce a new kind of matter and cannot be reversed. (ex.Cooking an Egg)
- Decomposition: One kind of matter decomposes to form 2 or more kinds of matter.
- Melting: Change of a solid to a liquid without the formation of a new kind of matter. (Ex. Melting ice.)
- Physical Changes: Changes that are easily reversed to get the original material back again.
2.7 Atoms p.36
- Atom: The smallest possible piece of something
- Not visible
- Idea of atoms in matter began in the 1800s

Thanks for reading. :)
Compounds and Elements 2.5 +2.8 summary
Now we all know that pure substances is made up of only one kind of matter and has a unique set of properties, such as; color, hardness, boiling point and melting point.
So, for example: If you were to add sand with water then its no longer a pure substance. The property changes and so does the mixture.
A pure substance is
Electrolysis is the production of a chemical reaction by means of an electric current.
Here's a video showing the electrolysis of water.
MORE facts about elements.
So, for example: If you were to add sand with water then its no longer a pure substance. The property changes and so does the mixture.
A pure substance is
- either an element or a compound.
Electrolysis is the production of a chemical reaction by means of an electric current.
Here's a video showing the electrolysis of water.
MORE facts about elements.
- it cannot broken down / decompose
- there are 109 elements
- it can exist as a solid, liquid, or gas
- can exist as an individual or as larger units
- have different melting and boiling points
- made of more than one atom
Finding Out about Matter (Summary of page 25-28)
Matter in the Miscroscopic World
How do you observe when you just look at the picture
How can you describe what you see so that others can understand results of careful observation?
These are the questions you will explore in taking a closer look at matter!
Science applies the same skills you use to interpret the picture to make increasingly presice observationand detailed interences. Detailed observation takes so much time and effort that it is necessary to speciallize.
Chemists specialize in matter; what it is, how one kind differs from another, what differencekinds have in common, how one kind can be changed to another, and even how mateer can be kept the same.
2-1 What You Know about Matter
By living in a world of matter, you have learned a tremendous amout about the subject. (ex. water normally exists as a liquid) A variety changes can be understood using a few powrful ideas may be even more amazing to you now. To see how a few ideas can explain a lots of observation, you will learn to specialize, you will look carefully at a few familiar substances , classify them, and make generalizations about them based on the reqularities you see.
Property
Mixture - Matter that is easily separated into component parts. It is impure.
Classifying matter into pure forms and mixtures is one step toward understanding matter and developing a language to describe that understanding. It is based on experience. Chemists derive the idea of mixture and pure substance to describe matter.
People purify water by adding alum and lime to the water, and a gelatinous material is produced. As this gelatinous material settles out or is removed by filtering through beds of sand, small particles suspended in the water stick to the gelatinous material and are removed.
Scattering cannot be used as a test of whether a material is pure. Mixtures like salt water or sugar water that look unifrom throughout and do scatter light are called solutions.
Salt water cna sugar water can be separated into their component parts using a procedure called destillation.
Although most mistures containing water can be separated by distillation, some cannot.
ex. household ammonia, whisky
Many mistures are difficult to separate . Such mixtures may be considered pure for many years until someone invents a new procedure or a better instrument for analyzing and separation. Many environmental conerns have come about because new and better techniques have been found to detect thier impurities.
How do you observe when you just look at the picture
How can you describe what you see so that others can understand results of careful observation?
These are the questions you will explore in taking a closer look at matter!
Science applies the same skills you use to interpret the picture to make increasingly presice observationand detailed interences. Detailed observation takes so much time and effort that it is necessary to speciallize.
Chemists specialize in matter; what it is, how one kind differs from another, what differencekinds have in common, how one kind can be changed to another, and even how mateer can be kept the same.
2-1 What You Know about Matter
By living in a world of matter, you have learned a tremendous amout about the subject. (ex. water normally exists as a liquid) A variety changes can be understood using a few powrful ideas may be even more amazing to you now. To see how a few ideas can explain a lots of observation, you will learn to specialize, you will look carefully at a few familiar substances , classify them, and make generalizations about them based on the reqularities you see.
Property
- characteristic of matter
- describe matter specifically and can be used to identify matter
- ex. colour, tast, boiling point
- part of the study of science is the accumulation of such information
- another part is using the information you have to make sense of the world by decribing it with increasingly greater precision
Mixture - Matter that is easily separated into component parts. It is impure.
Classifying matter into pure forms and mixtures is one step toward understanding matter and developing a language to describe that understanding. It is based on experience. Chemists derive the idea of mixture and pure substance to describe matter.
People purify water by adding alum and lime to the water, and a gelatinous material is produced. As this gelatinous material settles out or is removed by filtering through beds of sand, small particles suspended in the water stick to the gelatinous material and are removed.
Scattering cannot be used as a test of whether a material is pure. Mixtures like salt water or sugar water that look unifrom throughout and do scatter light are called solutions.
Salt water cna sugar water can be separated into their component parts using a procedure called destillation.
Although most mistures containing water can be separated by distillation, some cannot.
ex. household ammonia, whisky
Many mistures are difficult to separate . Such mixtures may be considered pure for many years until someone invents a new procedure or a better instrument for analyzing and separation. Many environmental conerns have come about because new and better techniques have been found to detect thier impurities.
Video: Classifying Matter and Pure Substances
Video: Solution and Classifying Matter
Subscribe to:
Posts (Atom)


















